EQA Schemes opened for registration
January 18, 2024
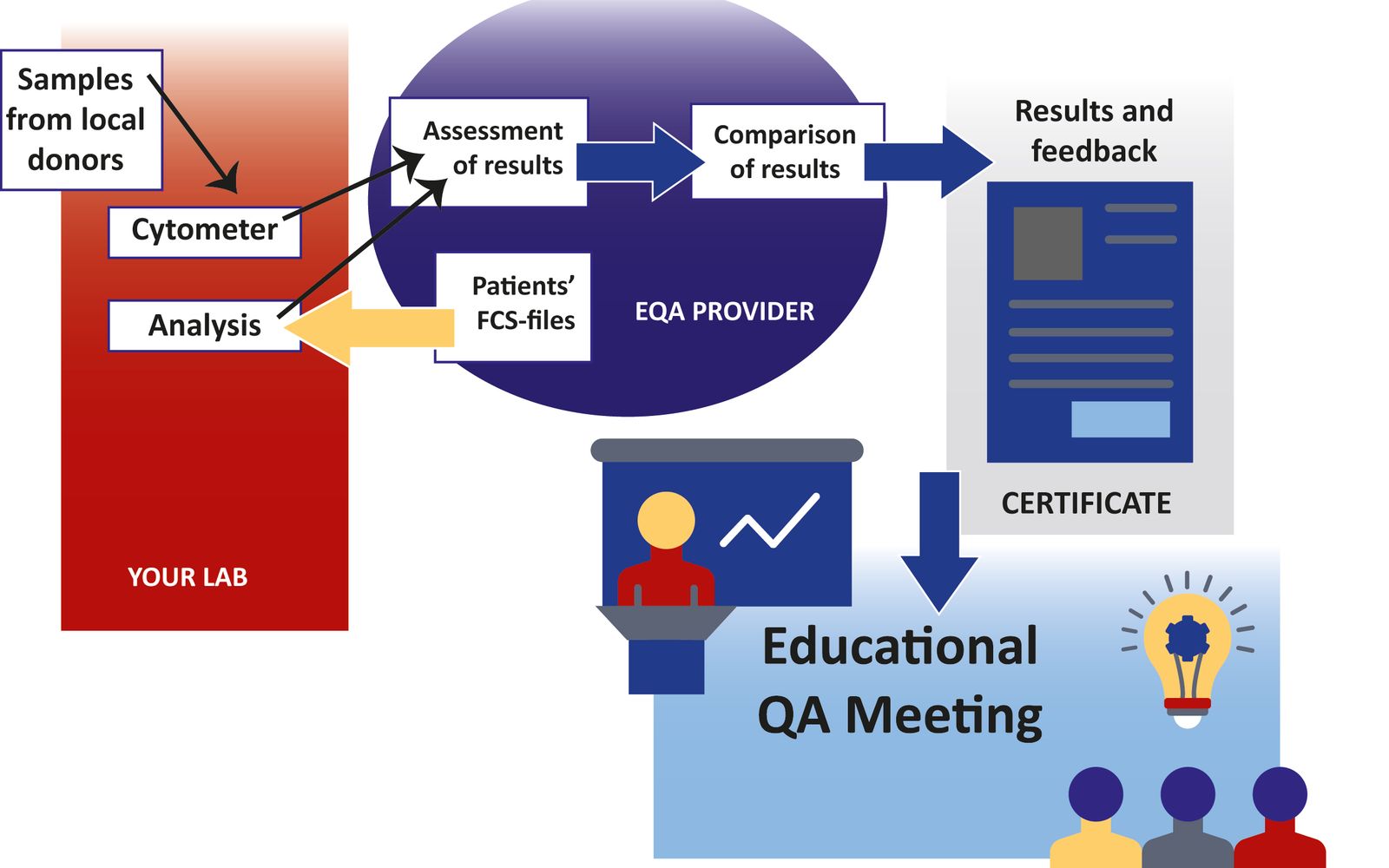
External Quality Assessment schemes are a tool for clinical laboratories to evaluate and manage the quality of laboratory practice with the support of an independent party.
The ESLHO EQA schemes help diagnostic laboratories worldwide to achieve the highest performance and quality. IG/TR clonality testing, MRD and flow cytometric immunophenotyping have become essential for an accurate diagnosis, classification, and disease monitoring in hemato-oncology. The EQA schemes answer the need for evaluation of performance, continuous improvement and education, and standardization/comparability of measurements in different diagnostic centers for these In Vitro Diagnostic tests.
Registration for the 2024 rounds of the EQA schemes of EuroClonality, EuroMRD and EuroFlow is now open. Do you wish to participate or want to learn more? Go to: eqascheme.org (IG/TR Clonality), www.euromrd.org (MRD) and/or www.euroflow.org/qa (FLOW) 📷 credit: Biomedical Quality Assurance Research Unit of the KU Leuven.
